Background: Acute myeloid leukemia (AML) is a cancer of the blood and bone marrow cells that requires intensive chemotherapy combined with hematopoietic stem cell transplantation in fit patients and less intensive treatment in elderly patients. Supportive care is also advised due to disease and therapy-related cytopenia that can lead to life-threatening systemic infections, bleeding, and hypoxemia.
Introduction: The Peripherally Inserted Central Catheter (PICC) may be a safe and useful tool for the treatment of AML patients because it is easier to implant and has a lower rate of infectious complications than the Centrally Inserted Central Catheters (CICC).
Methods: Since its establishment in our medical oncology department, a PICC team consisting of a hematology physician and three dedicated nurses has conducted a prospective study to evaluate the efficacy and complication rate of the PICC device in inpatient and outpatient AML patients. Inclusion criteria included all AML patients who required chemotherapy, supportive care, and HSCT, regardless of white blood cell (WBC) count. A platelet count greater than 20000/mm 3 and normal coagulation tests were required for implantation. In all patients, the vascular anatomy of the arms was previously examined by ultrasound. All implantations were performed under ultrasound guidance followed by radiographic control.
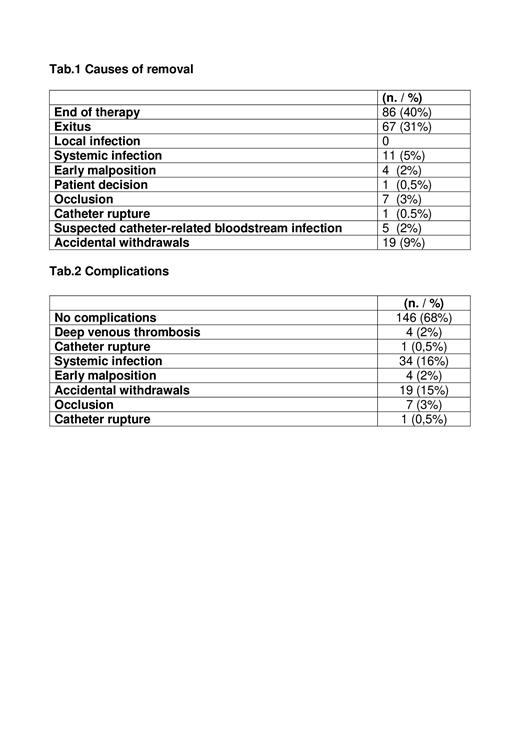
Results: From March 2007 to April 2023, 216 PICC implantations were performed in 186 patients (110 men and 76 women), including 13 patients with acute promyelocytic leukemia, after the conclusion of the induction phase. Median age 60 years (range 22-79). The majority of implantations were performed in hospitalized AML patients (193 - 89%). One hundred ninety-three PICCs (81%) were used for chemotherapy, thirty (14%) for supportive care, four (2%) for autologous HSCT, five (3%) for allogeneic HSCT. Only two PICCs were lost to follow-up in the AML population. Therefore, the analysis of duration and complications was performed on 214 devices. At the time of this analysis, 13 of 214 PICCs (6%) are still in situ and in use. The total duration of PICC life was 26289 days (median, 103; range, 0-959). The reasons for removal in the two populations are shown in Table1. Of note, most PICCs were removed because of end of therapy (40%) and exitus (31%), and only one in five (20%) had suspected catheter-related bloodstream infection confirmed by microbiological testing. Complications recorded in the global populations are described in the Table.2
No complications occurred in 68% of PICCs. There were 34 episodes of confirmed PICC-related septicemia recorded (16%; 1.3/1000 days/PICC). The pathogens isolated in blood cultures and PICC tip analysis were: Staphylococcus Epidermidis, Staphylococcus Hominis, Staphylococcus Warneri, Pseudomonas Aeruginosa, Escherichia Coli, Enterococcus Faecium, Enterococcus Fecalis, Stenotrophomonas Maltophila, Bacillus Cereus, Klebsiella Pneumoniae, Candida spp. In only 11 of 34 confirmed PICC-related septicemias (32%) did the devices need to be removed, whereas in the other 23 cases (68%), PICC were left in place after the infection resolved with systemic antibiotic therapy. There were only 4 (2%; 0.15/1000 days/PICC) cases of symptomatic thrombotic complications related to PICC without the need for removal.
Conclusions: These data indicate that the use of PICC can be considered a safe and useful tool in the treatment of AML patients because it is easy to insert, safe to use, long lasting, and has a low complication rate, even in the case of PICC-related systemic infections.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal